
Ozone is a gas. A toxic, pale blue, odorous, irritative and explosive gas. But without it being naturally formed and making up part of our atmosphere, we’d be unable to survive on Earth. We’d literally fry from the sun’s ultraviolet rays. The thin layer of ozone, which sits in the bottom part of the stratosphere about 9-18 miles above the Earth, absorbs approximately 98% of extremely harmful UVB rays, which are the most damaging of the ultraviolet types (A, B and C). So, way back in the 80’s when it was discovered that there was a ‘hole’ in this protective layer of gas over Australia, Antarctica and the Arctic, a massive global response was initiated.
Let’s take a step back to science class for a moment. What exactly is this gas? It’s actually a form of oxygen — with 3 atoms, instead of 2, so named O3. It is formed when O2 is split apart by sunlight to make single oxygen atoms. They can then re-join together to form O2, or join with O2 to make O3. It’s 1.5 times more dense than oxygen, turning to liquid at -112° C (-170° F) and freezing at -251.4° C (-420° F). For comparison, O2 freezes at -218.79° C (-361.82° F). The density and instability make ozone even more volatile than oxygen, meaning that although it can be manufactured by hand (and has many uses, such as a bleaching agent or germicide), the process is difficult and hazardous.
Apart from manufacturing the gas intentionally for commercial use, it is also produced anthropogenically via a photochemical reaction from nitrogen oxides (NOx, for example from vehicle exhaust) combining with volatile organic compounds (VOCs) in the presence of sunlight -— essentially as a result of air pollution. Examples of VOCs are formaldehyde, acetone and benzene, and range from having no health impact to being extremely toxic. When ozone is formed from anthropogenic activities, the ozone is ground-level, sitting within the troposphere rather than the stratosphere. And that means it’s dangerous for life on Earth rather than helpful. It also makes it a significant greenhouse gas.
So, if we’re both intentionally and unintentionally producing ozone, how could there be a hole in this layer of atmosphere? Firstly, I should clarify that it’s not actually a hole: it’s an area where the gas has thinned out. And it’s not technically ‘an area’, because the thinning is both seasonal and slightly transient! For example, during August to October, the Antarctic ‘hole’ is worst. But how did it happen? Molecules of ozone can absorb limited quantities of chlorine. Once a maximum threshold is reached, the chlorine atoms destroy the ozone molecules. In fact, one atom of chlorine can destroy 100,000 ozone molecules, so nature simply cannot produce them as quickly as we’re killing them off.

Where does this damaging chlorine come from then? Predominantly, chlorofluorocarbons or CFCs. The sun’s UV rays break down the CFCs to carbon, fluorine and chlorine. CFCs used to be in almost every aerosol spray can and refrigeration unit, until the monumental global movement which I referred to above. On September 16, 1987, one hundred and ninety seven countries, every member of the UN, signed the Montreal Protocol on Substances that Deplete the Ozone Layer. This treaty sits under the Vienna Convention for Protection of the Ozone Layer, and orchestrated the binding, well-planned, and monitored phasing out of all chemicals which are ozone depleting: CFCs, halons, and less damaging transitional chemicals such as hydrochlorofluorocarbons (HCFCs). The Protocol targets 96 ozone-depleting chemicals in thousands of applications across more than 240 industries. In 2016, the Montreal Protocol had a further add-on, the Kigali Amendment, and is now also responsible for setting binding progressive phase-down obligations for the production and use of the 18 main hydrofluorocarbons. Super interestingly, these HFCs, considered to be next generation of coolants, are not ozone depleting, but are effective heat-trapping greenhouse gases. So far, only 58 UN members have signed this Amendment.
And now for some good news! NASA has been measuring the ozone layer rigorously, and published a study in the beginning of 2018 confirmed a 20% drop in ozone depletion! It was also reported that at the projected rates, Northern Hemisphere and mid-latitude ozone is scheduled to heal completely by the 2030s, followed by the Southern Hemisphere thinning in the 2050s and polar regions by 2060. Based on HFCs being a GHG, the Assessment found the world can avoid up to 0.4°C of global warming this century through implementation of the Kigali Amendment, affirming its critical role in keeping global temperature rise below the 2°C mark.
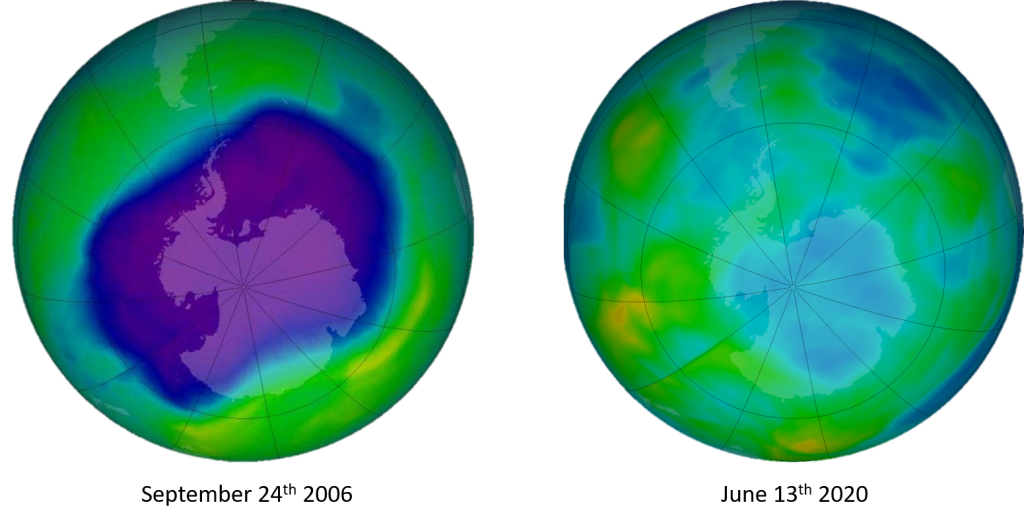
In another recent scientific study, published in Nature, it was found that the emissions of ozone depleting chemicals began to decline in the year 2000. And the thinning layer of ozone started to heal! That is, the natural process for ozone production started to catch up with the depletion of the molecules. All thanks to the Montreal Protocol. The larger super positive takeaway from this is that action such as this Protocol WORKS!
But this only takes care of the discussion around the ‘good’ ozone — the protective layer of molecules in the stratosphere. What about the troposphere ozone? For obvious reasons, this is referred to as the ‘bad’ ozone. It is formed through pollution and causes human health risks, sometimes very serious, as well as environmental damage. For people who are elderly or have asthma, exposure to raised levels of ozone can lead to death. But even healthy members of the community could experience coughing, throat irritation, chest tightness, wheezing, and burning sensation on deep breathing. For the environment, we’re looking at impacts on certain sensitive vegetation and ecosystems, especially during growing season, as photosynthesis is inhibited. Some examples of particularly sensitive plants are white pine, black cherry, soybean, spinach and aspen.

It’s important to understand the difference between the ‘good’ and ‘bad’ ozone or, rather, the good and bad location for the ozone molecules. And remember that the good ozone molecules are 100% naturally occurring. We are still working towards repairing the thinning ozone layer in the stratosphere, and we have to pay huge credit to the Montreal Protocol for the amazing progress made thus far. We now need continuing scientific work to create replacements for HFCs. Bearing in mind that the World Health Organization reported 4.2 million deaths across the globe in 2016 related to ambient air pollution, we need to ensure regulations and policies are put into place to limit our continued production of ‘bad’ ozone. On a personal level, we must monitor our local ozone levels, particularly for those in our community who are elderly or suffer from respiratory illness. The numbers to be on the lookout for are anything near or higher than 70 parts per billion with exposure for 8 hours. As a reference point, natural ozone concentration is about 10 parts be billion. Monitoring can be done for the United States through AIRNow or EnviroFlash; for Australia at Air-Quality; for Europe at the European Environment Agency; or globally at The World Air Quality Project.



